ATP-Binding Cassette (ABC) transporters work as molecular pumps to transport substrates across cellular membranes. Represented in all organisms, ABC proteins are divided into two classes (i) exporters that mediate the transport of unrelated substances across membranes and (ii) importers which function to allow nutrients into the cell. To date, 13 human diseases are linked to defective transporters, including the multidrug resistance (MDR) phenomenon. In order for us to combat the role these proteins play in diseases, deciphering the mechanism substrate specificity and the role of ATP in substrate translocation is of paramount importance.
My laboratory is currently conducting research in four areas:
- Transport mechanism. How do organisms utilize transporters to shuttle nutrients or toxins across membranes?
- Host-pathogen interaction: How do pathogens utilize ABC transporters to circumvent the host innate immune defenses?
- Transcriptional regulation of ABC transporters. What signals do transcriptional activators receive to turn on expression of specific transporters?
- Classification of ABC transporters: What is the role of regulatory domains in the transport process?
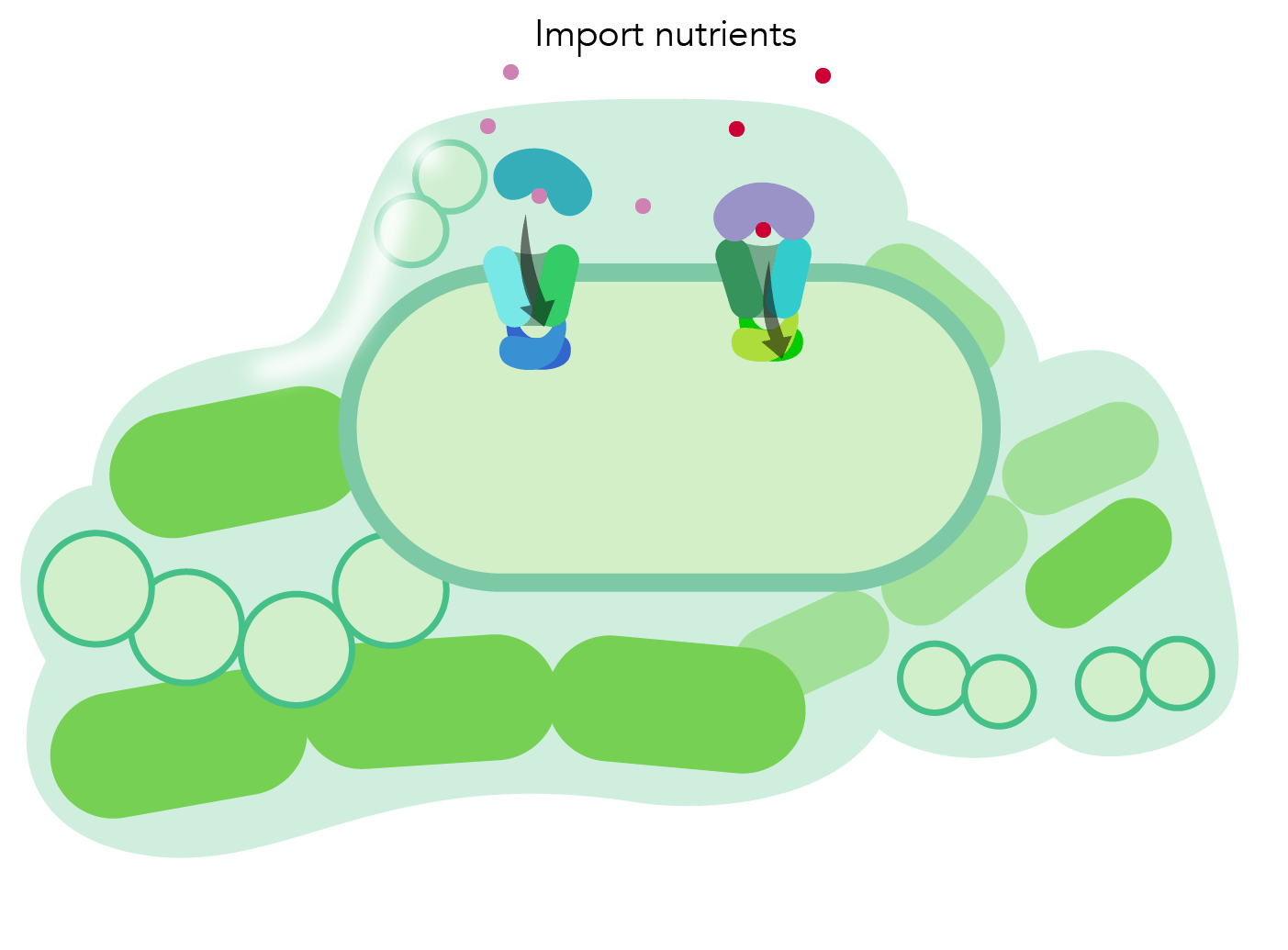 Transport mechanism: In order to understand the transport process, we study how both exporters and importers control the passage of nutrients, lipids, amino acids and toxins across lipid bilayers into the cell. Our studies of a type II molybdate transport from H. influenzae revealed how the helices in the transmembrane domains reposition themselves to allow a small compound to pass through. This work works indicates a novel gating mechanism for nutrient uptake in bacteria specific for small molecule transport. [Learn more here] Science illustration by Grace Hsu.
Transport mechanism: In order to understand the transport process, we study how both exporters and importers control the passage of nutrients, lipids, amino acids and toxins across lipid bilayers into the cell. Our studies of a type II molybdate transport from H. influenzae revealed how the helices in the transmembrane domains reposition themselves to allow a small compound to pass through. This work works indicates a novel gating mechanism for nutrient uptake in bacteria specific for small molecule transport. [Learn more here] Science illustration by Grace Hsu.
 Host Pathogen Interaction: Bacteria that commonly co-exist within its human host can become pathogenic causing disease. We are interested in how the bacteria avoid the human immune responses and thus prevent pathogen death. To understand this mechanism of resistance, we study the bacteria Non-typeable Haemophilus influenzae (NTHI), which exists in the nasal cavity of its human host scavenging nutrients like iron, but as a pathogen causes middle ear infection (otitis media). NTHI is able to avoid the effects of antimicrobial peptides by utilizing a specially adapted transport system, abbreviated “Sap” for its sensitivity to antimicrobial peptides, to shuttle host-derived antimicrobial peptides into cellular compartments for destruction. We are interested in understanding how the ABC transporter SapABCD recognizes and shuttles antimicrobial peptides into the cellular space for destruction. [Learn more here] Cover by graduate student Kristen Rivera
Host Pathogen Interaction: Bacteria that commonly co-exist within its human host can become pathogenic causing disease. We are interested in how the bacteria avoid the human immune responses and thus prevent pathogen death. To understand this mechanism of resistance, we study the bacteria Non-typeable Haemophilus influenzae (NTHI), which exists in the nasal cavity of its human host scavenging nutrients like iron, but as a pathogen causes middle ear infection (otitis media). NTHI is able to avoid the effects of antimicrobial peptides by utilizing a specially adapted transport system, abbreviated “Sap” for its sensitivity to antimicrobial peptides, to shuttle host-derived antimicrobial peptides into cellular compartments for destruction. We are interested in understanding how the ABC transporter SapABCD recognizes and shuttles antimicrobial peptides into the cellular space for destruction. [Learn more here] Cover by graduate student Kristen Rivera
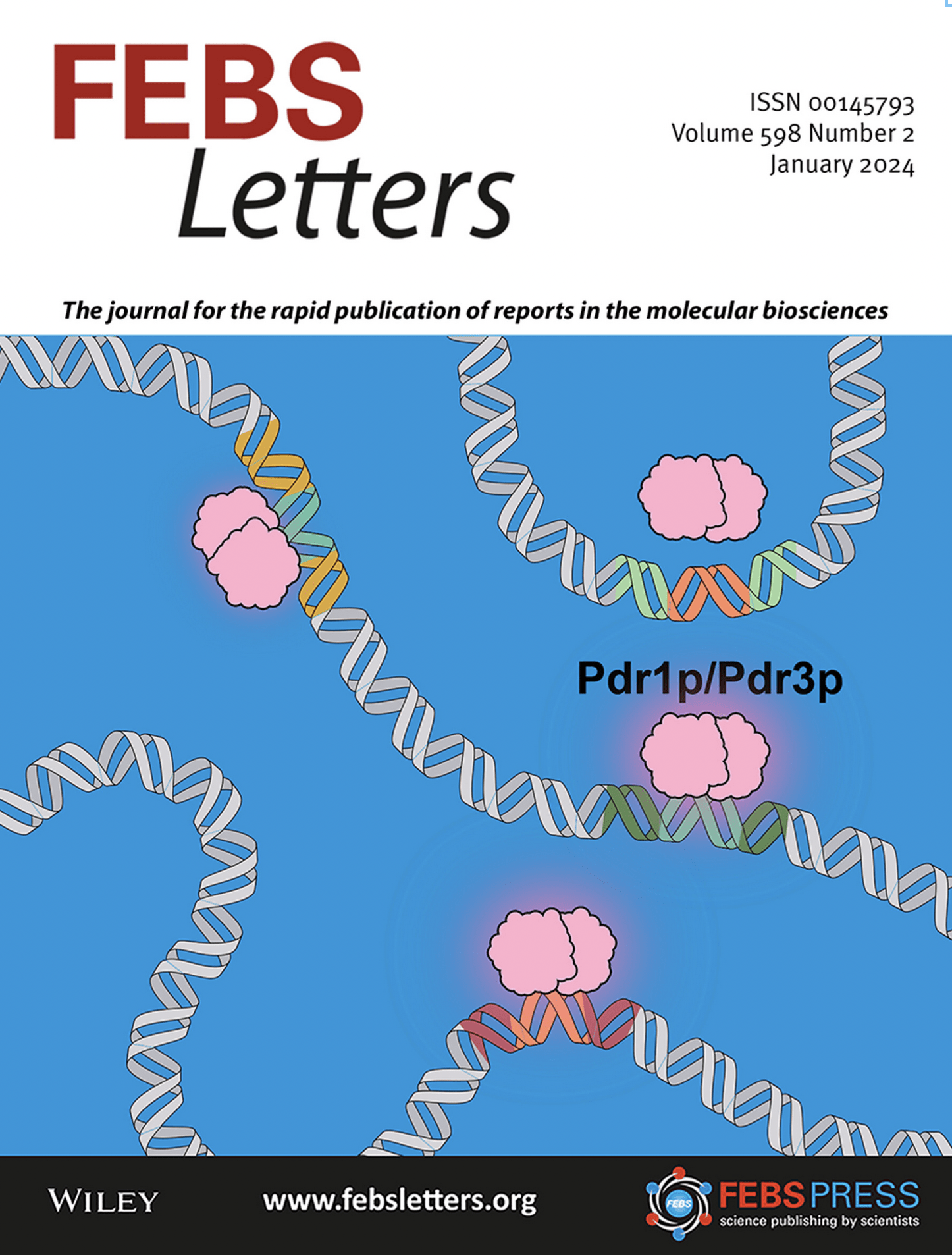 Transcriptional regulation of ABC transporters: Since ABC transporters recognize and export a range of chemicals, it makes sense to implement strategies to regulate their overexpression. In S. cerevisiae, overexpression of efflux pumps is controlled by transcriptional regulators of the PDR network Pdr1p and Pdr3p. Pdr1p/Pdr3p regulate expression of certain PDR exporters by binding to consensus motifs called pleiotropic drug-resistance elements (PDREs). These regulators form dimers and work in concert by recognizing a series of perfect CGG repeats in the promoter region of target genes. [Learn more here] Cover art by graduate student Evan Buechel
Transcriptional regulation of ABC transporters: Since ABC transporters recognize and export a range of chemicals, it makes sense to implement strategies to regulate their overexpression. In S. cerevisiae, overexpression of efflux pumps is controlled by transcriptional regulators of the PDR network Pdr1p and Pdr3p. Pdr1p/Pdr3p regulate expression of certain PDR exporters by binding to consensus motifs called pleiotropic drug-resistance elements (PDREs). These regulators form dimers and work in concert by recognizing a series of perfect CGG repeats in the promoter region of target genes. [Learn more here] Cover art by graduate student Evan Buechel
Classification of ABC importers: Structures of ABC transporters have revealed a divergence in overall architecture that is not quite understood. For example, high-resolution structures of importers show a variable number of helices in the transmembrane domains as well as the presence or absence of a cytosolic regulatory domain. Since these accessory domains are not conserved in all ABC importers, the idea of two subfamilies of ABC importers came into play, Type I and Type II ABC transporters.
Rivera K.G., Tanaka K.J., Buechel E.R, Origel O. Jr, Harrison A., Mason K.M., Pinkett H.W. (2024) Antimicrobial Peptide Recognition Motif of the Substrate Binding Protein SapA from Nontypeable Haemophilus influenzae. Biochemistry. 63(3):294-311.
Buechel E.R, Pinkett H.W. (2023) Activity of the pleiotropic drug resistance transcription factors Pdr1p and Pdr3p is modulated by binding site flanking sequences. FEBS Lett. 598(2):169-186.
Dimitrova, V.S.,Song, S., Karagiaridi, A., Marand, A., Pinkett, H.W. (2021) Detergent Alternatives: Membrane Protein Purification Using Synthetic Nanodisc Polymers. Methods in Molecular Biology. 2507:375-387.
Thomas, C., Aller, S. G., Beis, K., Carpenter, E. P., Chang, G., Chen, L., Dassa, E., Dean, M., Duong Van Hoa, F., Ekiert, D., Ford, R., Gaudet, R., Gong, X., Holland, I. B., Huang, Y., Kahne, D. K., Kato, H., Koronakis, V., Koth, C. M., Lee, Y., Lewinson, O., Lill, R., Martinoia, E., Murakami, S., Pinkett, H. W., Poolman, B., Rosenbaum, D., Sarkadi, B., Schmitt, L., Schneider, E., Shi, Y., Shyng, S. L., Slotboom, D. J., Tajkhorshid, E., Tieleman, D. P., Ueda, K., Varadi, A., Wen, P. C., Yan, N., Zhang, P., Zheng, H., Zimmer, J., and Tampe, R. (2020) Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett. 594(23):3767-3775
Buechel, E. R., and Pinkett, H. W. (2020) Transcription factors and ABC transporters: from pleiotropic drug resistance to cellular signaling in yeast. FEBS Lett. Dec;594(23):3943-3964.
Tanaka KJ, Pinkett H.W. (2019) Oligopeptide-binding protein from nontypeable Haemophilus influenzae has ligand-specific sites to accommodate peptides and heme in the binding pocket. J Biol Chem 2019, 294(3):1070-1082
Hardison R.L., Harrison A., Wallace R.M., Heimlich D.R., O’Bryan M.E., Sebra R.P., Pinkett H.W., Justice S.S., Mason K.M (2018). Microevolution in response to transient heme-iron restriction enhances intracellular bacterial community development and persistence. PLoS, 14(10):e1007355.
Tanaka, K.J., Song, S., Mason, K., Pinkett, H.W. (2018). Selective substrate uptake: The role of ATP-binding cassette (ABC) importers in pathogenesis. Biochim Biophys Acta. 1860(4): 868-877
Rice, A.J., Park, A., and Pinkett, H.W. (2014). Diversity in ABC Transporters: Type I, II and III Importers. Critical Reviews in Biochemistry and Molecular Biology 49(5): 426-37Rice A.J., Alvarez F.J., Davidson A.L., Pinkett H.W. (2014). Effects of lipid environment on the conformational changes of an ABC importer. Channels. 22;8(4).
Rice A.J., Alvarez F.J., Davidson A.L., Pinkett H.W. (2014). Small substrate transport and mechanism of a molybdate ATP binding cassette transporter in a lipid environment. Journal of Biological Chemistry 289(21):15005-13.
Rice A.J., Alvarez F.J., Schultz K.M., Klug C.S., Davidson A.L., Pinkett H.W. (2013). EPR Spectroscopy of MolB2C2-A Reveals Mechanism of Transport for a Bacterial Type II Molybdate Importer. Journal of Biological Chemistry. 288(29):21228-35.
Commentary for this article: Paper of the Week, top 2% of JBC papers in overall importance.
Tirado-Lee, L.M., Lee. A.T., Rees, D.C., Pinkett, H.W. (2011). Classification of a Haemophilus influenzae ABC transporter HI1470/71 through its cognate molybdate periplasmic binding protein, MolA. Structure 19, 1-10.
Commentary for this article: Type II ABC permeases: are they really so different? (2011) George AM, Jones PM. Structure.19, 1540-2.
Pinkett, H. W., Lee, A. T., Lum, P., Locher, K. P., & Rees, D. C. (2007). An inward-facing conformation of a putative metal-chelate ABC transporter. Science. 315, 373-377.
Faculty of 1000 evaluation: New finding.
Pinkett, H. W., Shearwin, K. S., Stayrook, S., Dodd, I., Burr, T., Hochschild, A., Egan, J., & Lewis, M. (2006). The Structural Basis of Cooperative Regulation at an Alternate Genetic Switch. Molecular Cell. 21(5), 605-615.
Pollock, P. M., Cohen-Solal, K., Sood, R., Namkoong, J., Martino, J. J., Koganti, A., Zhu, H., Robbins, C., Makalowska, I., Shin, S. S., Marin, Y., Roberts, K. G., Yudt, L. M., Chen, A., Cheng, J., Incao, A., Pinkett, H.W., Graham, C. L., Dunn, K., Crespo-Carbone, S. M., Mackason, K. R., Ryan, K. B., Sinsimer, D., Goydos, J., Reuhl, K. R., Eckhaus, M., Meltzer, P. S., Pavan, W. J., Trent, J. M., & Chen, S. (2003). Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nature Genetics. 34(1),108-12.